Despite the abundance of evidence-based methods for treating tobacco use, research indicates that nearly half of the patients who visited a healthcare provider in the past year were not advised to quit smoking, and the majority of those who attempted to quit were not offered evidence-based cessation treatment. This article explores a quality improvement project that translated the latest evidence-based recommendations into practice to enhance the screening and treatment of tobacco users at a rural primary care clinic. A standardized, evidence-based protocol that utilized existing, free resources was implemented and assessed over three months. Results were compared to baseline data from the three months prior to the practice change. Screening, treatment, and follow-up for tobacco users improved after the QI project. Patient screening increased from 80.1% at baseline to 99.4% post-intervention, while tobacco cessation interventions by providers for active smokers rose from 63.6% to 90.1%. The authors discuss how nurses can apply findings from this QI project to enhance their use of evidence-based tobacco cessation interventions in daily practice and assist patients in quitting smoking.
Key Words: tobacco cessation, smoking cessation, quality improvement, primary care, health disparities, evidence-based practice, nursing, nurse practitioner, quit smoking, smoking interventions, translational research
Smoking is a public health epidemic responsible for nearly 500,000 deaths in the United States every year (Centers for Disease Control and Prevention, 2020). The latest U.S. Surgeon General’s Report on tobacco use indicates that although smoking prevalence rates are declining—13.7% for cigarettes and 19.7% for all tobacco products in 2018—more than 34 million people in the U.S. still smoke (USDHHS, 2020). Significant disparities in smoking prevalence exist across the U.S. based on age, race, sex, socioeconomic status, and geographic location (Table 1), with certain groups, such as those with lower socioeconomic status, smoking at a rate twice the national average (USDHHS, 2020).
Tobacco use is the leading preventable cause of morbidity and mortality in the U.S. and represents a significant issue for healthcare providers to address in their daily practice. Tobacco exposure leads to disease through four primary pathologic mechanisms: lung damage, cancer, vascular disease from the promotion of atherosclerosis and plaque instability, and inflammation that results in a weakened and dysfunctional immune system (Davis, 2019). These pathological changes can occur in individuals who do not Tobacco use is the leading preventable cause of morbidity and mortality in the U.S. and represents a significant issue for healthcare providers to address in their daily practice.smoke but have been exposed to secondhand and thirdhand smoke, which includes contact with residual nicotine and other chemicals that cling to surfaces like clothing or walls after a cigarette is extinguished. This exposure also encompasses smoke from e-cigarettes (Davis, 2019; USDHHS, 2020). Annually, secondhand smoke is estimated to cause 3,400 lung cancer deaths and 42,000 heart disease deaths in individuals who never smoked (USDHHS, 2014). Children and infants are particularly vulnerable; exposure to smoke increases the risk of Sudden Infant Death Syndrome (SIDS), asthma, acute respiratory infections, and ear infections (Davis, 2019).
Table 1. Tobacco Use Prevalence in High-Risk Populations
|
Lower socioeconomic status |
26.2% |
|
High school education or below |
41.4% |
|
Males |
25.8% |
|
Uninsured |
29.9% |
|
Medicaid |
27.9% |
|
Psychiatric or substance abuse diagnosis |
36.7% |
|
American Indian/Alaskan Natives |
32.3% |
|
Non-Hispanic multiracial adults |
25.4% |
|
Homosexual or bisexual adults |
29.2% |
|
Midwest |
23.6% |
|
Southern U.S. |
24.1% |
Note. The 2020 Smoking Cessation Report from the U.S. Surgeon General outlined several high-risk groups that have a smoking prevalence of 20% or more—table based on data from Creamer et al., 2019; Heck, 2019; USDHHS, 2020.
Background and Significance
The majority of people who smoke want to quit—an estimated 68% of U.S. adults—but only a small percentage actually succeed in becoming tobacco-free (USDHHS, 2020). Data from a 2015 national health survey indicated that 55% of smokers made a quit attempt in the past year, but only 7% were successful (Babb et al., 2017). Those who try to quit without the aid of smoking cessation resources are even less likely to succeed (USDHHS, 2020). Standard tobacco cessation practices focus on combining pharmacotherapy with behavioral therapy (Barua et al., 2018; Fiore et al., 2008; Kruger et al., 2016; Stead et al., 2016). However, recent research indicates that individuals who may wish to quit smoking are not receiving any advice or treatment from their healthcare providers. Seventy percent of smokers visit a healthcare provider each year (Rice et al., 2017). Nevertheless, 44% of adult smokers who saw a healthcare provider in the last year were not advised to quit, and 2 out of 3 adults who attempted to quit smoking in that period did not utilize evidence-based treatment (USDHHS, 2020). The majority of people who smoke want to quit—an estimated 68% of U.S. adults—but only a small percentage actually succeed in becoming tobacco-free.
These findings highlight the necessity for healthcare providers to be well-versed in evidence-based smoking cessation treatments and to feel confident in implementing them in practice, particularly for high-risk groups. Research indicates that advice from healthcare providers regarding the risks of smoking and available options for quitting serves as a strong motivator for patients attempting to quit (Aveyard et al., 2011; Garcia-Gomez, 2019; Kruger et al., 2016; Wu et al., 2016). Numerous studies have confirmed the safety and effectiveness of three pharmacological treatments for smoking cessation: nicotine replacement therapy (NRT), bupropion, and varenicline (Cahill et al., 2015; Cinciripini et al., 2018; Howes et al., 2020). Among non-pharmacological treatment methods, psychological interventions have the greatest supporting evidence, with individual behavioral strategies, such as relaxation exercises and self-observation, proving to be the most effective. Other effective treatments include brief counseling, cognitive-behavioral therapy, and telephone counseling (Garcia-Gomez et al., 2019). Combining pharmacotherapy with counseling has been shown to double the likelihood of success in quitting smoking (Garcia-Gomez et al., 2019). Guidelines from the U.S. Department of Health and Human Services, the Agency for Health Care Policy and Research, and the U.S. Public Health Service recommend a smoking cessation strategy that integrates pharmacotherapy and behavioral interventions.
Need for Quality Improvement in Screening and Treatment of Tobacco Users
The care team at a primary care clinic serving a rural, underserved population in the Southern U.S. observed high rates of tobacco use among its patients. Many had several comorbid conditions affected by tobacco use, including peripheral arterial disease, heart disease, COPD, asthma, hypertension, and diabetes. The clinic also treated numerous patients with mental health and substance abuse disorders—demographics known to have a high risk of tobacco use. Despite strong evidence supporting the effectiveness of combined pharmacotherapy and behavioral interventions for smoking cessation, these methods were not regularly implemented in the clinic. Additionally, there was a lack of standardization in screening for tobacco use, treatment for cessation, and a failure to utilize existing resources.
The purpose of this quality improvement project was to translate the latest evidence-based recommendations into practice to enhance the clinic’s screening and treatment processes for patients using tobacco products. Our primary objective was to develop a standardized protocol for use in low-resource settings that utilized existing free resources and established a process applicable regardless of which provider treated the patient. Another goal was to integrate the screening and treatment protocol into the clinic’s electronic health record (EHR) to promote meaningful use. Our project aligned with the clinic’s goals of delivering quality, cost-effective care to patients while increasing the screening and treatment of tobacco users, which is a meaningful use requirement assessed by the Centers for Medicare and Medicaid Services (CMS) and utilized to determine reimbursement.
Literature Review
To guide this practice change, a literature review was conducted using the following PICOT question: For adult smokers at a rural primary care practice, does implementing an evidence-based screening and treatment protocol, compared to usual care, increase the screening of patients for tobacco use and the provider's use of pharmacological and behavioral smoking cessation interventions during a 12-week period? The following key terms were used: smoking cessation interventions, tobacco abuse treatment, primary care, ambulatory care, nurse, and adult. Subsequent subject headings and title searches were also employed to identify potential articles. These terms were searched in the CINAHL, PubMed, and Cochrane Library databases. A search of Google Scholar was also conducted to identify grey literature that might be overlooked during database searches. Inclusion criteria for articles in the review included research studies published in peer-reviewed journals between 2015 and 2020, written in English, and addressing tobacco cessation interventions in an outpatient setting. Articles focusing on inpatient interventions and those published in other languages were excluded. A few seminal works, treatment guidelines, and government reports published before 2015 were also considered. The articles and studies reviewed primarily consisted of meta-analyses, systematic reviews, and randomized controlled trials. A small number of well-designed quasi-experimental and observational studies were also included, along with recommendations from evidence-based treatment guidelines and government reports.
Key Literature Findings
Strong, high-quality evidence supports the use of a combined pharmacological and behavioral strategy as the standard of care for tobacco cessation (Barua et al., 2018; Fiore, 2000; Kruger et al., 2016; Stead et al., 2016). For primary care providers, pharmacotherapy combined with counseling or other behavioral treatments has proven to be clinically and cost-effective (Ekpu and Brown, 2015). The literature also endorses activities such as assessing tobacco use status, providing at least brief advice on cessation, and offering willing patients evidence-based treatment at every office visit (Aveyard et al., 2011; Garcia-Gomez et al., 2019; Kruger et al., 2016; Vidrine et al., 2013; WHO, 2014). Moderate evidence exists for stage-matched cessation interventions and nurse-led initiatives (Gokbayrak et al., 2014; Lu et al., 2019; Rice et al., 2017). The literature strongly indicates disparities in tobacco treatment, and efforts should emphasize reaching high-risk groups (Nemeth et al., 2018; Smith et al., 2019; USDHHS, 2020). Emerging interventions, such as social media use, EHR alerts, and gene-guided treatment, show promise but require further high-quality studies with large sample sizes (Bae et al., 2018; Naslund et al., 2016; Wells et al., 2018). With fewer than one-third of tobacco users employing FDA-approved pharmacotherapy or behavioral interventions during a quit attempt (USDHHS, 2020), a more standardized approach is necessary to address tobacco use in outpatient settings. This indicates that more needs to be accomplished to effectively translate evidence-based interventions for tobacco cessation into practice settings.
Conceptual and Theoretical Framework
The theoretical framework selected to guide this quality improvement project was the Transtheoretical Model of Behavior Change (TTM), which outlines five stages of change people experience as they modify their behavior: precontemplation, contemplation, preparation, action, and maintenance (Prochaska et al., 1992). Developed by Prochaska et al. (1992), the model was initially designed to understand how patients independently change addictive behaviors and with professional assistance. It was based on research conducted with thousands of patients who were trying to change addictive behaviors such as smoking, obesity, and alcohol abuse. The model integrates processes of change from various psychotherapy theories. Now validated, it is widely used and applied to various behaviors, including dietary and exercise habits, smoking cessation, substance abuse, and contraceptive use (Zimmerman et al., 2000).
The model emphasizes that a person’s stage of change affects their readiness to modify a behavior, influenced by their Decisional Balance, Situational Temptations, and Process of Change activities (Gokbayrak et al., 2014). In the first three stages, patients evaluate the pros and cons of making a change, with success relying on their conviction that the change will be beneficial. In the last two stages, success increasingly rests on their confidence in the ability to change, which is influenced by their self-efficacy, environmental resources, and perceived support (Rochette et al., 2009). Successful change depends on taking the right actions at the right time (Prochaska et al., 1992). In practical applications, this involves first assessing a patient’s readiness to change according to the five stages of change and customizing interventions to fit the stage. Figure 1 presents a diagram of key concepts and an adapted application of the TTM used for this project.
Figure 1. Transtheoretical Model of Behavior Change: Key Concepts and Clinician Interventions
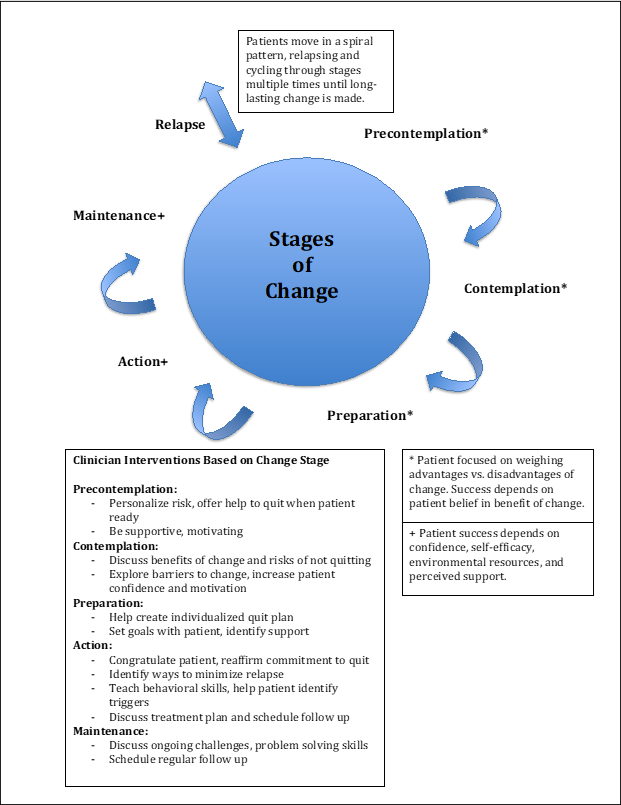
Note. Adapted model created from information from AAFP (2017), Prochaska et al. (1992), Rochette et al. (2009), and Zimmerman et al. (2000).
Methodology
The Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines were utilized to organize this quality improvement project, as one of our objectives was to establish a standardized screening and treatment protocol applicable at other low-resource clinics. The SQUIRE guidelines offer a framework to guide QI efforts to ensure results can be shared meaningfully (Ogrinc et al., 2015). Change tests within Plan, Do, Study, Act (PDSA) cycles were employed to assess the practice change. This facilitated improvement through a step-wise approach: planning changes, testing them, analyzing results, and modifying based on what was successful and what was not (Institute for Healthcare Improvement [IHI], 2017). During implementation, the QI team conducted weekly meetings with the clinic staff to report progress and gather feedback on the efficacy of the new process.
Since this was a quality improvement project implementing evidence-based educational and treatment interventions, no IRB approval was needed. The clinic also did not require IRB approval, but the quality improvement team and clinic staff created and signed a detailed plan for maintaining patient privacy and confidentiality during this project. All data collected from the clinic’s EHR was stored securely using REDCap; only de-identified data was used, and results were presented in aggregate with no specific identifiers.
Setting and Participants
This quality improvement project was carried out at a rural health clinic in the Southern U.S. that primarily serves socioeconomically disadvantaged patients, many of whom are uninsured or receive Medicaid. This clinic sees between 30 and 50 patients daily from Monday through Friday. It is owned by a cardiology physician and staffed with three family nurse practitioners, three medical assistants, two front office staff members, an office manager, and a registered nurse who manages referrals and post-procedure care. Patients receive primary care, urgent care, and cardiology services at this multi-specialty practice. The clinic has a high prevalence of smoking among adults, with 38.7% of patients identified as active tobacco users.
As part of the patient intake process, all patients seeking primary and urgent care at the clinic were screened for tobacco use. Those who were identified as tobacco users were counseled on smoking cessation using the 5A framework, offered FDA-approved treatment, and scheduled for follow-up or given a referral to the state’s Quitline, a free telephone service providing counseling and support for tobacco cessation. All clinic providers and support staff participated in education and training on the tobacco screening and treatment protocol.
Intervention
This QI project had two phases. In the first phase, clinic staff received training on the dangers of tobacco use, evidence-based recommendations for screening and treatment, and new workflows employing a standardized protocol for patient screening, treatment, and follow-up. Two training sessions were conducted: one for support staff that concentrated on screening and follow-up, and another for providers that focused on evidence-based counseling and treatment. Providers were instructed to assess a patient’s stage of change and customize counseling accordingly. During the second phase, practice changes were enacted, and staff adhered to a standardized protocol for patient screening, treatment, and follow-up (Figure 2).
Figure 2. Key Workflow Steps in Tobacco Screening and Treatment Protocol
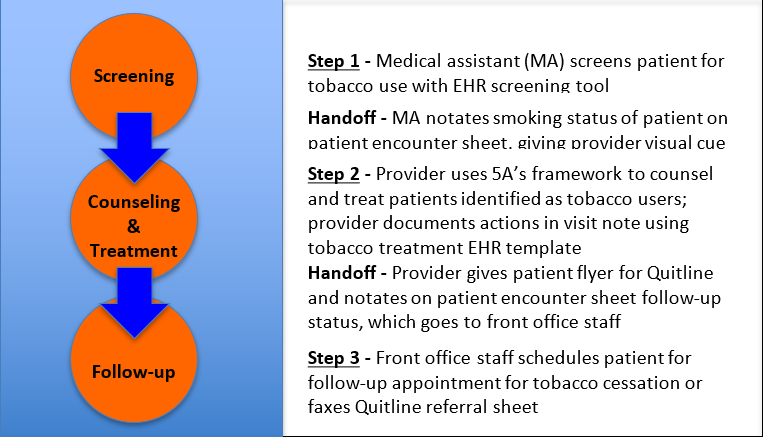
Screening
The initial protocol had three steps. First, medical assistants (MAs) carried out universal tobacco screening of all patients coming into the clinic using the clinic’s EHR screening tool (Figure 3). MAs were taught to treat tobacco use status as a vital sign to be documented at every visit and shown how to complete the tobacco screening form in the EHR. The clinic had not previously used this full tobacco-screening tool during patient intake.
Figure 3. Electronic Health Record Tobacco Use Screening

Counseling and Treatment
The next step involved counseling and treatment for individuals identified as active tobacco users. Providers utilized the evidence-based 5A’s framework to discuss tobacco use and encourage cessation with patients during each visit. This intervention is designed for use by busy clinicians in an office setting and can be completed in 3 to 5 minutes. It includes asking about tobacco use; advising all tobacco users to quit with a clear, strong, and personalized message; assessing a patient’s readiness to attempt quitting; assisting patients who are prepared to quit by helping them create a quit plan, providing counseling, and offering medications; and arranging appropriate follow-up for the patient or referring them to specialty services (WHO, 2014).
All providers received a 5A’s pocket card as a reference, a free online resource, to assist with counseling. For patients who expressed readiness to quit, providers offered an FDA-approved pharmacological treatment along with brief behavioral counseling that included setting a quit date and strategies for managing cravings. Those who were not yet ready to quit received counseling \using motivational interviewing appropriate to their stage in the Transtheoretical Model (TTM) of change and were offered a referral to follow up with the clinic’s tobacco treatment provider. Providers then recorded that they provided counseling and/or treatment to patients identified as tobacco users using an EHR documentation template. Before the project, the clinic had not established any EHR documentation templates for tobacco counseling or treatment.
Follow-up
The final step in the tobacco screening and treatment protocol involved scheduling follow-up appointments for active tobacco users. Based on provider recommendations, the front office staff scheduled patients for follow-up appointments specifically for tobacco treatment or faxed a patient referral sheet to Quitline, which offers free tobacco cessation services, including telephone counseling and free nicotine replacement products. Additionally, the front office staff maintained a list to track patients referred for tobacco treatment, facilitating follow-up and data collection.
Change Tests
After several PDSA cycles, we identified several key process changes and modified the screening and treatment protocol. One significant change was the implementation of handoffs with visual cues to enhance the transfer of information between MAs, providers, and front office staff. This linked the steps of the protocol and improved compliance. In addition to completing the tobacco screening in the EHR, the MAs noted the tobacco use status at the top of the patient’s encounter sheet, which was presented to the providers at the beginning of the visit. This visual cue allowed providers to quickly identify tobacco users, enabling them to plan their counseling and treatment accordingly. Another essential handoff was from the provider to the front office staff for scheduling follow-ups. As we adjusted the protocol, we recognized the need for a more straightforward, patient-centered follow-up process. Besides faxing referrals to the Quitline, we started providing patients with a one-page handout that included information about the Quitline and how to access its resources independently. This resulted in increased follow-up rates and reduced the chance of losing contact with patients whose phone numbers had changed or been disconnected.
Data Collection and Analysis
Using a National Quality Forum (NQF) approved measure, we evaluated the percentage of patients aged 18 and older screened for tobacco use during the data collection period and who received tobacco cessation interventions if identified as tobacco users (NQF, 2020). We also assessed the proportion of eligible patients scheduled for follow-up tobacco treatment or referred to the Quitline. Data were collected through chart reviews for three months at baseline and three months following the implementation of the QI program. REDCap was utilized to gather and store data and analyze the results. A total of 360 charts were reviewed—180 at baseline and 180 post-intervention. We conducted a descriptive analysis to evaluate the collected data, examining frequencies, measures of central tendency, and percentages.
Goals for measuring success were based on the Healthy People 2020 targets for smoking cessation: increasing tobacco screening in office-based outpatient settings to 83.3% and boosting tobacco cessation counseling in outpatient settings to 12.2% (Office of Disease Prevention and Health Promotion [ODPHP], 2020). As data was collected, it was entered into run charts to track and evaluate the results, enabling the QI team to determine whether the interventions used in each PDSA cycle were achieving the success measures. While collecting and tabulating data, we met weekly with an interdisciplinary team from the clinic, including representatives from leadership, providers, MAs, and front desk staff, to review and interpret the results.
Results
Patients involved in the quality improvement project were between age 18 and 89. The majority of tobacco product users preferred cigarettes (95%, n=95), while 3% (n=3) used chewing tobacco and 1% (n=1) used vapes or e-cigarettes. Most of the participants had a diagnosis of hypertension (84.4%, n=206), followed by diabetes (36.1%, n=88), and nearly 50% (n=120) had asthma, COPD, or hyperlipidemia. Most visits were for primary care (60.8%, n=219), with 29.2% (n=105) in urgent care, and 20% (n=36) via telehealth. Of the 180 charts reviewed at baseline, 33 did not include an assessment of tobacco use status. Among those assessed for tobacco use at baseline (n=147), 51.7% (n=76) had never used tobacco, 10.9% (n=16) were former tobacco users, and 37.4% (n=55) were current tobacco users. In the 180 charts reviewed post-intervention, 179 (99.4%) included an assessment of tobacco use, showing that 32.4% (n=58) had never used tobacco products, 27.9% (n=50) were former users, and 39.7% (n=71) were actively using tobacco products.
Field notes taken during the baseline data collection revealed several notable observations. Few patients had complete tobacco screenings. Most charts included documentation from providers of only basic counseling, such as "discussed the risks of smoking” and "strongly advised the patient to quit smoking.” None of the providers counseled patients who indicated they used chewing tobacco. These patients were also not scheduled for follow-up specifically for smoking cessation or referred to resources like the Quitline. Very few patients received medications for smoking cessation, and those who did often did not receive evidence-based treatments. One example was using nicotine gum or patches as monotherapy rather than dual nicotine replacement therapy (NRT), which is the standard of care. Few providers offered counseling alongside medication therapy, which is also the current standard of care. Additionally, most did not assess a patient’s readiness to quit, which is important for tailoring a personalized tobacco cessation message.
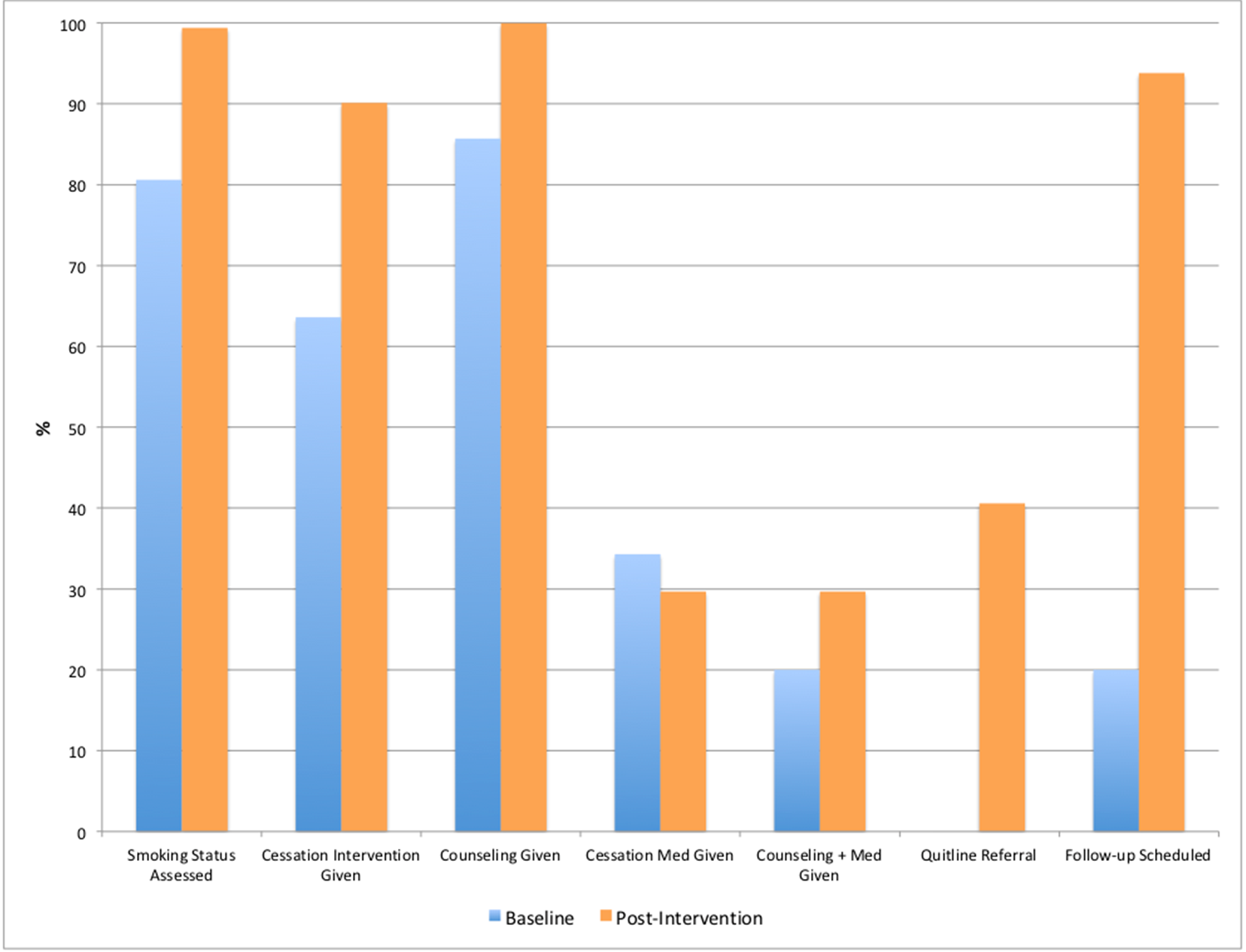
The QI program enhanced screening, treatment, and follow-up for patients who use tobacco products (Table 2). Comparing baseline data to post-intervention data revealed a significant increase in patient screening for tobacco use, rising from 80.1% (n=145) to 99.4% (n=179). Post-intervention tobacco screening assessments were also twice as likely to include details about the type of tobacco product used and the number of cigarettes smoked daily. Furthermore, more patients were assessed for their readiness to quit during their tobacco use screening, increasing from 5.6% (n=10) at baseline to 27.2% (n=49) after the intervention. The QI program enhanced screening, treatment, and follow-up for patients who use tobacco products.
For active smokers, tobacco cessation interventions provided by healthcare professionals increased from 63.6% (n=35) to 90.1% (n=64). Post-intervention, counseling was provided 100% (n=64) of the time, compared to 85.7% (n=30) at baseline. Fewer tobacco cessation medications were prescribed after the intervention; 12 of 35 active smokers (34.3%) received cessation medications at baseline, versus 19 of 64 active smokers (29.7%) after the QI project was implemented. The provision of counseling and education—the current standard of care—rose from 20% (n=7) at baseline to 29.7% (n=19) post-intervention. Follow-up for these patients also improved, with 40.6% (n=26) of patients receiving referrals to the Quitline post-intervention. No patients from the baseline group were referred to the Quitline. In the post-intervention group, 93.8% (n=60) of patients receiving tobacco cessation treatment were scheduled for a follow-up, compared to 20% (n=7) at baseline.
Table 2. Key Findings from Tobacco Screening and Treatment QI Program
Discussion
This quality improvement project significantly increased universal patient screening for tobacco use and enhanced the quality of those screenings, including assessments of readiness to quit and the type and amount of tobacco products used. Improvements were also observed in provider treatment of patients identified as active tobacco users, with increases in counseling, counseling combined with medication use, and referrals for follow-up. A key change in practice was the utilization of the Quitline for follow-up, a free telephone-based service that offers counseling and support for tobacco cessation. Prior to the implementation of the QI program, this resource was not utilized at the clinic. These results align with findings in the literature (Flocke et al., 2019; Jansen et al., 2014; LeLaurin, 2020).
Another significant practice change was the implementation of the 5A’s framework to enhance provider counseling, standard-of-care treatment, and follow-up for patients actively using tobacco products. After the intervention, all identified tobacco users received cessation counseling, a greater number of patients were provided with standard-of-care treatment that included both medication and counseling, and follow-up appointments for tobacco use increased more than fourfold compared to baseline. This aligns with findings from other studies in the literature, which indicate that patients receiving all 5A’s were considerably more likely to utilize counseling and medications for tobacco cessation (Aveyard et al., 2011; WHO, 2014; Kruger et al., 2016).
A challenge during the implementation phase was managing staff turnover, particularly during the COVID-19 pandemic when conditions were changing weekly. We discovered that the staff needed frequent re-education on the new workflows for screening, counseling, and follow-up. To address this issue, we incorporated tobacco screening and treatment protocols into the new staff orientation education, as previously shown in a study by Jansen et al. (2014) to enhance the sustainability of practice changes from a tobacco cessation QI program. We also designated one staff member, our registered nurse, as the education champion for the program. She was responsible for re-education activities and for gathering staff feedback on the new process. The implementation of EHR-integrated screening and treatment facilitated improved program uptake and documentation compliance, aligning with findings from the literature (Flocke et al., 2019; Jansen et al., 2014; LeLaurin et al., 2020; Rindal et al., 2013).
An unexpected finding was that the percentage of individuals who had never used tobacco products decreased post-intervention from 51.7% (n=76) to 32.4% (n=58), while the number of former tobacco users rose from 10.9% (n=16) to 39.7% (n=50). This shift likely occurred because the quality of screening assessments improved, leading to more former smokers being identified in the post-intervention phase. Rather than categorizing individuals as nonsmokers, more patients were recognized as former smokers. The literature indicates that capturing this information enables better relapse monitoring and more treatment interventions by providers during patient visits (Fiore et al., 2008; Gökbayrak et al., 2015).
Another unexpected finding was a decrease in the number of tobacco cessation medications prescribed after the intervention. Although providers documented more counseling activities and combined counseling with medications, their prescribing practices did not significantly change. This trend has been observed in other research, including a large study conducted at Veterans Affairs medical centers that examined organizational interventions to enhance the use of evidence-based guidelines for tobacco screening and treatment (Joseph et al., 2004). A possible explanation is that providers in this quality improvement study remained uncomfortable with tobacco treatment medications, and the education provided was inadequate to alter their prescribing habits. This is supported by a study by Lawvere et al. (2005), which explored nurse practitioners’ knowledge of tobacco screening and cessation. Researchers found that nurse practitioners could effectively apply counseling for tobacco cessation but had a limited understanding of first-line pharmacotherapy treatments and how to manage high-risk patients. One approach to overcoming this barrier would be to offer more comprehensive training on prescribing tobacco treatment medications or to conduct follow-up education sessions to reinforce previous learning and provide feedback on current practices. Andrews et al. (2001) demonstrated that offering feedback to primary care clinicians improved their adherence to tobacco treatment guidelines.
Strengths, Limitations, and Sustainability
The primary strength of this quality improvement project lies in its use of existing, free resources and simple workflow changes to establish a standardized protocol. This allows the program to be implemented with minimal capital investment from a clinic, making it ideal for low-resource environments. The main costs associated with this QI program included staff training time and printed materials for both patients and staff. This protocol can be integrated into an existing clinic workflow with only a slight increase in time commitment. The 5A’s framework is designed for use within 3 to 5 minutes, while the EHR tobacco use screening requires about 1 minute to complete. Furthermore, another strength is that data was compared at baseline and post-intervention over a 6-month period, providing insight into results over time.
Limitations included that only patients who spoke English participated in the program. The program did not improve the extent to which providers prescribed evidence-based treatments. Data was gathered solely through chart reviews, which could introduce bias since the information was documented retrospectively. Finally, the program was tested at only one clinic with a small staff.
One issue that may affect the sustainability of this practice change is staff turnover. To address this, the clinic can incorporate tobacco screening and treatment protocol education into its orientation and training for all new employees. Another way to make this project sustainable is to appoint a project champion at the clinic. This person will be responsible for the maintenance of the program and collecting data intermittently to ensure that program goals continue to be met.
Implications for Nursing Practice
There are several takeaways from this quality improvement program that nursing professionals can use to improve screening and treatment in their practice or when designing a similar program at their clinic. Most importantly, interventions and workflow changes must be simple and patient-centered. For example, when we started giving patients a handout for the Quitline, this eliminated the delay in follow-up and decreased the chances that someone would be missed due to a missing or incorrect phone number. Another takeaway is the importance of addressing tobacco use status at every visit and providing at least brief advice to those identified as active tobacco users, recommendations supported by the Agency for Healthcare Research and Quality (AHRQ) Clinical Practice Guideline for Treating Tobacco Use and Dependence (Fiore et al., 2008). Many patients needed repeated encouragement before they decided to make a cessation attempt. Tobacco use is a chronic, relapsing condition. Thus, it is also important to identify patients who formerly used tobacco so they can be monitored for relapse and offered screening for tobacco-related conditions, such as lung cancer. Furthermore, robust evidence indicates that patients age 18-24 are more susceptible to relapse and require additional support following tobacco cessation.
Integrating screening, counseling, and treatment documentation into the EHR improves staff compliance with the protocol and documentation. This can be done using existing EHR tools. For this QI program, we worked with representatives from the clinic’s EHR vendor to customize the tobacco-screening tool, create documentation templates, and set up clinical decision support alerts for staff. Visual cues are also important for facilitating workflow changes, especially during handoffs between team members. In our clinic, the MAs noted and highlighted the smoking status of the patient at the top of the patient’s encounter form that was given to the nurse practitioner (NP) prior to the visit. This reminded the NP to address tobacco use during each visit. NPs were not always going to the EHR between visits and would miss tobacco use information. NPs were given 5A’s pocket cards that summarized the steps in the counseling and treatment process, another form of clinical decision support.
A future direction for this project could focus on evidence-based strategies to enhance clinician treatment of identified tobacco users. Another significant direction would be to implement this program across a multi-clinic system, including patients who speak different languages. Data could be gathered on follow-up numbers to determine if patients referred to the Quitline utilized the resource and whether it influenced their attempt to quit. Finally, evidence-based modifications to improve patient outcomes after they initiate treatment for tobacco cessation could be explored, including the use of nurse-led interventions and social media.
Conclusion
The results of this quality improvement project demonstrate the effectiveness of a low-cost, evidence-based protocol for enhancing screening, treatment, and follow-up for patients using tobacco products in a rural primary care clinic environment. Nurses working in low-resource outpatient settings can utilize this model to bridge gaps in the screening and treatment process for patients using tobacco products, thereby helping more patients successfully quit smoking. Findings from this project illustrate the feasibility and acceptability of these practice changes. Furthermore, this standardized protocol can be implemented in a relatively short time with minimal capital investment, as it relies on free online resources such as the state’s Quitline and the 5A’s framework.
Authors
Rachael L. Joyner, DNP, APRN, FNP-BC, NCNTT
Email: Rachael.Joyner@duke.edu
Rachael L. Joyner, DNP, APRN, FNP-BC, NCNTT, is a family nurse practitioner specializing in primary care, tobacco cessation treatment, and preventive medicine. Dr. Joyner received her BSN and MSN from Florida Atlantic University and her DNP from the University of Florida where she did quality improvement research on tobacco treatment in rural primary care settings. She holds a National Certificate in Nicotine and Tobacco Treatment and currently works as a tobacco treatment specialist with the Duke Smoking Cessation Program, which is part of the Duke University Health System.
Sandra W. Citty, PhD, APRN-BC, FNP-BC, CNE-BC
Email: swolfe@ufl.edu
ORCID ID: https://orcid.org/0000-0002-4769-7760
Dr. Citty has 30 years experience in the nursing profession as a clinician, educator, and scientist. Dr. Citty received her Bachelor’s and Master’s Degrees in Nursing from the University of Miami (Coral Gables, Florida) and her PhD in Nursing from the University of Florida. Dr. Citty’s scholarship focuses on quality and process improvements to enhance healthcare and patient outcomes. Dr. Citty currently serves as a Clinical Associate professor at the University of Florida College of Nursing and is a Clinical Nurse Investigator in the Geriatric Research Education Clinical Center (GRECC) and North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Jane M. Carrington, PhD, RN, FAAN
Email: janemcarrington@outlook.com
Jane M. Carrington, PhD, RN, FAAN, has 26 years of experience in informatics, as an analyst and scientist. Her research focus is nurse-to-nurse communication for a patient with a change of condition using the electronic health record. The change in condition of particular interest is a clinical event. Dr. Carrington has mentored numerous DNP and PhD students and taught evidence-based practice and quality improvement and informatics courses. Dr. Carrington has retired from University of Florida and now works on projects advancing artificial intelligence and nursing informatics in nursing and nursing education.
Tanya Gibbs, MSN, APRN-BC
Email: tanyagibbsfnp@gmail.com
Tanya Gibbs, MSN, APRN-BC, is a board-certified family nurse practitioner. She completed her BSN at Fayetteville State University and her MSN as a Family Nurse Practitioner from South University in Savannah, GA. She has worked as a Nurse Practitioner in Family Medicine, Urgent Care, and Weight Loss management with over 20 years of experience in healthcare. Her career goal is to help others become the healthiest version of themselves, which includes work treating patients for tobacco use. She currently owns her own practice, Awaken Wellness NC, specializing in wellness and medical weight loss services.
References
American Academy of Family Physicians. (2017). Treating tobacco dependence practice manual: A systems-change approach. https://www.aafp.org/patient-care/public-health/tobacco-nicotine/tools/toolkit.html
Andrews, J. O., Tingen, M. S., Waller, J. L., & Harper, R. J. (2001). Provider feedback improves adherence with AHCPR Smoking Cessation Guideline. Preventive Medicine, 33(5), 415–421.
Aveyard, P., Begh, R., Parsons, A., & West, R. (2011). Brief opportunistic smoking cessation interventions: A systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction, 107, 1066-1173. https://doi.org/10.1111/j.1360-0443.2011.03770.x
Babb, S., Malarcher, A., Schauer, G., Asman, K., & Jamal, A. (2017). Quitting smoking among adults — United States, 2000–2015. Morbidity Mortality Weekly Report, 65, 1457–1464. https://doi.org/10.15585/mmwr.mm6552a1.
Bae, J., Ford, E. W., Kharrazi, H. H. k., & Huerta, T. R. (2018). Electronic medical record reminders and smoking cessation activities in primary care. Addictive Behaviors, 77, 203-209. https://doi.org/10.1016/j.addbeh.2017.10.009
Barua, R. S., Rigotti, N. A., Benowitz, N. L., Cummings, K. M., Jazayeri, M-A, Morris, P.B., Ratchford, E. V., Sarna, L., Stecker, E. C., Wiggins, B. S. (2018). ACC expert consensus decision pathway on tobacco cessation treatment. Journal of the American College of Cardiology, 73, 3332–3365. https://doi.org/10.1016/j.jacc.2018.10.027
Cahill, K., Stevens, S., Perera, R., & Lancaster, T. (2015). Pharmacological interventions for smoking cessation: An overview and network meta‐analysis. Cochrane Database of Systematic Reviews(5). https://doi.org/10.1002/14651858.CD009329.pub2
Centers for Disease Control and Prevention. (2020, Dec. 10). Current cigarette smoking among adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
Cinciripini, P. M., Minnix, J. A., Green, C. E., Robinson, J. D., Engelmann, J. M., Versace, F., Wetter, D. W., Shete, S., & Karam-Hage, M. (2018). A RCT with the combination of varenicline and bupropion for smoking cessation: Clinical implications for front line use. Addiction. https://doi.org/10.1111/add.14250
Creamer, M. R., Wang, T. W., Babb, S., Cullen, K. A., Day, H., Willis, G., Jamal, A., & Neff, L. (2019). Tobacco product use and cessation indicators among adults — United States, 2018. Morbidity Mortality Weekly Report, 68(45), 1013–1019. https://doi.org/10.15585/mmwr.mm6845a2
Davis, J. (2019). Medical and psychometric testing. In J. Greyber (Ed.), Duke-UNC tobacco treatment specialist training program: 2019 training manual (pp. 5-15). Fairfield, OH: Duke-UNC.
Ekpu, V. U., & Brown, A. K. (2015). The economic impact of smoking and of reducing smoking prevalence: Review of evidence. Tobacco Use Insights, 8, 1-35. https://doi.org/10.4137/tui.s15628
Fiore, M. C. (2000). A clinical practice guideline for treating tobacco use and dependence: A U.S. Public Health Service Report. JAMA, 283(24), 3244-3254. https://jamanetwork.com/journals/jama/fullarticle/192834
Fiore, M. C., Jaén, C. R., Baker, T.B., Bailey, W. C., Benowitz, N. L., Curry, S. J., Dorfman, S. F., Froelicher, E. S., Goldstein, M. G., Healton, C. G., Henderson, P. N., Heyman, R. B., Koh, H. K., Kottke, T. E., Lando, H. A., Mecklenburg, R. E., Mermelstein, R. J., Mullen, P. D., Orleans, C. T., …Wewers, M. E. (2008). Treating tobacco use and dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service.
Flocke, S. A., Seeholzer, E., Lewis, S. A., Gill, I. J., Ordillas, E., Rose, J. C., Albert, E., Love, T. E., & Kaelber, D. C. (2019). Designing for sustainability: An approach to integrating staff role changes and electronic health record functionality within safety-net clinics to address provision of tobacco cessation care. The Joint Commission Journal on Quality and Patient Safety, 45(12), 798-807. https://doi.org/10.1016/j.jcjq.2019.09.003.
Garcia-Gomez, L., Hernandez-Perez, A., Noe-Diaz, V., Riesco-Miranda, J. A., & Jimenez-Ruiz, C. (2019). Smoking cessation treatments: Current psychological and pharmacological options. Revista de Investigacion Clinica, 71, 7-16. https://doi.org/10.24875/RIC.18002629
Gökbayrak, N. S., Paiva, A. L., Blissmer, B. J., & Prochaska, J. O. (2015). Predictors of relapse among smokers: Transtheoretical effort variables, demographics, and smoking severity. Addictive Behaviors, 42, 176-179. https://doi.org/10.1016/j.addbeh.2014.11.022
Heck, C. (2019). Population data on tobacco use and tobacco use disparities. In J. Greyber (Ed.), Duke-UNC tobacco treatment specialist training program: 2019 training manual (pp. 26-48). Fairfield, OH: Duke-UNC.
Howes, S., Hartmann‐Boyce, J., Livingstone‐Banks, J., Hong, B., & Lindson, N. (2020). Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews, (4). https://doi.org/10.1002/14651858.CD000031.pub5
Institute for Healthcare Improvement. (2017). QI essentials toolkit: PDSA worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx
Jansen, A. L., Capesius, T. R., Lachter, R. Greenseid, L. O., & Keller, P. A. Facilitators of health systems change for tobacco dependence treatment: a qualitative study of stakeholders’ perceptions. BMC Health Serv Res 14, 575 (2014). https://doi.org/10.1186/s12913-014-0575-4
Joseph, A. M., Arikian, N. J., An, L. C., Nugent, S. M., Sloan, R. J., & Pieper, C. F. (2004). Results of a randomized controlled trial of intervention to implement smoking guidelines in Veterans Affairs medical centers: Increased use of medications without cessation benefit. Medical Care, 42(11), 1100–1110. https://pubmed.ncbi.nlm.nih.gov/15586837/
Kruger, J., O’Halloran, A., Rosenthal, A. C., Babb, S. D., Fiore, M. C. (2016). Receipt of evidence-based brief cessation interventions by health professionals and use of cessation assisted treatments among current adult cigarette-only smokers: National adult tobacco survey, 2009-2010. BMC Public Health, 16(141). https://doi.org/10.1186/s12889-016-2798-2
Lawvere, S., Mahoney, M. C., Englert, J. J., Murphy, J. M., Hyland, A., Klein, S. B., & Loewen, G, M. (2005). Nurse practitioners’ knowledge, practice and attitudes about tobacco cessation and lung cancer screening. Journal of the American Academy of Nurse Practitioners, 15(8), 376-381. https://doi.org/10.1111/j.1745-7599.2003.tb00411.x
LeLaurin, J. H., Theis, R. P., Thompson, L. A., Tan, A., Young-Wolff, K. C., Carter-Harris, L., Shenkman, E. A., & Salloum, R. G. (2020). Tobacco-related counseling and documentation in adolescent primary care practice: Challenges and opportunities. Nicotine & Tobacco Research. Official Journal of the Society for Research on Nicotine and Tobacco, 22(6), 1023–1029. https://doi.org/10.1093/ntr/ntz076
Lu, C. C., Hsiao, Y. C., Huang, H. W., Lin, J. Y., & Huang, C. L. (2019). Effects of a nurse-led, stage-matched, tailored program for smoking cessation in health education centers: A prospective, randomized controlled trial. Clinical Nursing Research, 28(7), 812-829. https://doi.org/10.1177/1054773817754276
Naslund, J. A., Kim, S. J., Aschbrenner, K. A., McCulloch, L. J., Brunette, M. F., Dallery, J., Bartels, S. J., & Marsch, L. A. (2017). Systematic review of social media interventions for smoking cessation. Addict Behav, 73, 81-93. https://doi.org/10.1016/j.addbeh.2017.05.002
National Quality Forum. (2020, February 10). Preventive care and screening: Tobacco use—screening and cessation intervention. https://www.qualityforum.org/
Nemeth, J. M., Thomson, T. L., Lu, B., Peng, J., Krebs, V., Doogan, N. J., Ferketich, A. K., Post, D. M., Browning, C. R., Paskett, E. D., & Wewers, M. E. (2018). A social-contextual investigation of smoking among rural women: Multi-level factors associated with smoking status and considerations for cessation. Rural Remote Health, 18(1), 4338. https://doi.org/10.22605/rrh4338
Office of Disease Prevention and Health Promotion. (2020, July 17). Healthy People 2020: Tobacco use objectives. https://www.healthypeople.gov/2020/topics-objectives/topic/tobacco-use/objectives
Ogrinc, G., Davies, L., Goodman, D., Batalden, P. B., Davidoff, F., Stevens, D. (2015). SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence: Revised publication guidelines from a detailed consensus process. BMJ Quality and Safety, 25, 986-992. https://doi.org/10.1136/bmjqs-2015-004411
Prochaska, J. O., DiClemente, C. C., & Norcross, J. C. (1992). In search of how people change: Applications to addictive behaviors. American Psychologist, 47(9), 1102-1114. https://psycnet.apa.org/fulltext/1993-09955-001.pdf
Rice, V., Heath, L., Livingstone‐Banks, J., & Hartmann‐Boyce, J. (2017). Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews, (12). https://doi.org/10.1002/14651858.CD001188.pub5
Rindal, B., Rush, W. A., Schleyer, T. K., Kirshner, M., Boyle, R. G., Thoele, M. J., Asche, S. E., Thyvalikakath, T., Spallek, H., Durand, E., Enstad, C. J., & Huntley, C. L. (2013). Computer-assisted guidance for dental office tobacco-cessation counseling: A randomized controlled trial. American Journal of Preventive Medicine, 44(3), 260-264. https://doi.org/10.1016/j.amepre.2012.10.023
Rochette, A., Korner-Bitensky, N., & Thomas, A. (2009) Changing clinicians' habits: Is this the hidden challenge to increasing best practices? Disability and Rehabilitation, 31(21), 1790-1794. https://doi.org/10.1080/09638280902803773
Smith, P., Poole, R., Mann, M., Nelson, A., Moore, G., & Brain, K. (2019). Systematic review of behavioural smoking cessation interventions for older smokers from deprived backgrounds. BMJ Open, 9(11), e032727. https://doi.org/10.1136/bmjopen-2019-032727
Stead, L. F., Koilpillai, P., Fanshawe, T. R., Lancaster, T. (2016). Combined pharmacotherapy and behavioral interventions for smoking cessation. Cochrane Database of Systematic Reviews, 3. Art. No.: CD008286. https://doi.org/10.1002/14651858.CD008286.pub3
U.S. Department of Health and Human Services. (2020). Smoking Cessation: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014.
Vidrine, J.I., Shete, S., Cao, Y., Greisinger, A., Harmonson, P., Sharp, B., Miles, L., Zbikowski, S. M., Wetter, D. W. (2013). Ask-Advise-Connect: A new approach to smoking treatment delivery in health care settings. JAMA Intern Med, 173(6), 458–464. https://doi.org/10.1001/jamainternmed.2013.3751
Wells, Q. S., Freiberg, M. S., Greevy, R. A., Tyndale, R. F., Kundu, S., Duncan, M. S., King, S., Abney, L., Scoville, E., Beaulieu, D. B., Greevy, R. A., Jr., Gatskie, V., & Tindle, H. A. (2018). Nicotine metabolism-informed care for smoking cessation: A pilot precision RCT. Nicotine & Tobacco Research, 20(12), 1489-1496. https://doi.org/10.1093/ntr/ntx235
World Health Organization. (2014). Toolkit for delivering the 5A’s and 5R’s brief tobacco interventions in primary care. WHO Press. https://www.who.int/tobacco/publications/smoking_cessation/9789241506953/en/
Wu, Q., Gilbody, S., Peckham, E., Brabyn, S., & Parrott, S. (2016). Varenicline for smoking cessation and reduction in people with severe mental illnesses: Systematic review and meta-analysis. Addiction, 111(9), 1554-1567. https://doi.org/10.1111/add.13415
Zimmerman, G. L., Olsen, C. G., & Bosworth, M. F. (2000). A ‘stages of change’ approach to helping patients change behavior. American Family Physician, 61(5), 1409-1416. https://www.aafp.org/afp/2000/0301/p1409.html


