When the mRNA COVID-19 vaccines were announced in December 2020 the world was excited that a vaccine was available to combat the coronavirus pandemic. One of the most frequent comments was a desire to wait because the vaccine technology was “so new.” This article will concentrate on the mRNA vaccines not familiar to the public and is intended to explain the developmental timeline before and after the genome of COVID-19 was announced. We discuss Operation Warp Speed and SARS-CoV-2 and specifically the development of Messenger RNA (mRNA) vaccines and concurrent other types of vaccines. Other topics of discussion include COVID-19 variants; effectiveness of mRNA vaccines; and late news about the Pfizer-BioNTech® COVID-19 vaccine. The article conclusion discusses implications for nurses as they continue to follow future developments, become competent in communicating viral epidemiology, and educate patients and families about vaccine options.
Key Words: COVID-19, mRNA, Pfizer®, Moderna®, vaccine technology, spike protein, SARS, MERS, self-amplification, COVID-19 variants, virus, booster doses, ACIP, patient education
Subsequent investigations identified this new virus as resembling SARS and MERS, but with some differencesIn late 2019 health officials noticed a new circulating virus. The World Health Organization (WHO) announced an infection in Wuhan, China on January 9, 2021 (AJMC Staff, 2021). At first it was considered a “new” virus but by the end of January 2020 the novel coronavirus had been identified in a patient traveling from China (AJMC Staff, 2021). Most readers are familiar with the common cold (caused by coronaviruses), and many have read about the Severe Acute Respiratory Syndrome (SARS) virus outbreak in 2003 and Middle East Respiratory Syndrome (MERS) in 2012. No additional SARS outbreaks have been noted since. Subsequent investigations identified this new virus as resembling SARS and MERS, but with some differences (Healthline, 2020).
In 2003, SARS was reported in Asia and subsequently resulted in 8,098 cases and 774 deaths. (Centers for Disease Control and Prevention [CDC], 2017). During the outbreak it spread to over 24 nations in North America, South America, Europe, and Asia. The spread was facilitated via international travel (CDC, 2017). The outbreak was controlled via aggressive public health measures (Morens et al., 2020) and antibiotics (Rat, Olivier, & Dutot, 2020) to address associated infections. MERS was reported in 2012 on the Arabian Peninsula (CDC, 2019) and continues to be monitored.
The COVID-19 virus spread more easily and rapidly than did the SARS virusSARS is a member of the coronavirus family, affecting humans and largely seen in bats (Healthline, 2020). MERS, another member of the coronavirus family, is found in humans and camels (CDC, 2015). SARS-CoV-2 (the COVID-19 virus) evolved from the SARS virus. It was first identified in late 2019 (Morens et al., 2020), spread quickly in early 2020 and was declared a pandemic by the WHO in March 2020 (WHO, 2020). According to Morens et al. (2020), the COVID-19 virus was most likely transmitted to humans either by bats or another host, such as pangolins found in Asia. The COVID-19 virus spread more easily and rapidly than did the SARS virus (Healthline, 2020).
Operation Warp Speed and SARS-CoV-2
Techniques included work on vaccine technology, and funding for vaccine manufacturing and distribution concurrent with clinical trials...The focus of treatment for the first SARS outbreak in 2003 was administration of antibiotics to treat bacterial infection (Rat et al., 2020). In 2020, the medical and public health community focused on development of vaccines as well as treatment with antivirals and stay-at-home and quarantine measures (Rat et al., 2020). To stimulate concentrated research along a variety of pathways, Operation Warp Speed was quietly started in early 2020 when the Trump administration designated around $10 billion in funding originally meant for hospitals and healthcare providers (Cohrs, 2021). This public-private partnership was officially announced on May 15, 2020 (U.S. Department of Defense [DoD], 2020) and supported several vaccine technologies to greatly accelerate results. Techniques included work on vaccine technology, and funding for vaccine manufacturing and distribution concurrent with clinical trials to hasten the development process (U.S. Government Accountability Office [GAO], 2021). Among the research platforms supported were the messenger RNA (mRNA), the Replication-defective-live-vector platform, and the Recombinant-subunit-adjuvanted protein platform. A brief description of each platform follows.
The mRNA platform, the basis of the Moderna® and the Pfizer/BioNTech® vaccine uses genetic spike RNA instructions from the SARS-CoV-2 virus. These instructions facilitate individuals to produce the spike protein of the COVID-19 virus and stimulate the body’s immune system to make the spike protein and promote the development of antibodies but cannot cause the COVID-19 disease (GAO, 2021).
The Replication-defective live-vector platform uses a non-replicating adenovirus to deliver genetic coding that facilitates the vaccinated person to produce antibodies but cannot cause the COVID-19 disease. The Johnson & Johnson Janssen® vaccine is an example of this technology (GAO, 2021).
Finally, the Recombinant-subunit-adjuvanted protein platform, developed by Novavax® and Sanofi/GCK starts with a fully-formed SARS-CoV-2 spike protein delivered to the individual along with an adjuvant – a product that helps stimulate the immune system but cannot cause COVID-19 (GAO, 2021). The Novavax® vaccine was submitted for EUA approval to the FDA on January 31, 2022 (Novavax, 2022). Since the initial announcement Novavax® announced in late February their intentions to pursue full approval by the FDA in the second half of 2022 (Reuters, 2022).
...the COVID-19 genetic sequence was released on January 11, 2020, with development of potential vaccines starting immediately.This article will concentrate on the mRNA vaccines not familiar to the public and is intended to explain the developmental timeline before and after the genome of COVID-19 was announced from a patient in Wuhan, China in January 2020 (Wu et al., 2020) and documented in Genbank, an international repository (Branswell, 2021a). According to Branswell, the COVID-19 genetic sequence was released on January 11, 2020, with development of potential vaccines starting immediately. With the release of the genetic code of the virus and the speed of the spread of the virus, vaccine developers focused on the protein spike on the virus exterior as the best target of a vaccine (Branswell, 2021a).
The Interest in the Spike Protein
...prior research on the SARS-CoV and MERS-CoV viruses noted structural similarities on the surface to the SARS-CoV-2 outer envelope. Salvatori et al. (2020) described how prior research on the SARS-CoV and MERS-CoV viruses noted structural similarities on the surface to the SARS-CoV-2 outer envelope. Salvatori discussed how the spike protein (S) was “highly immunogenic and protective” toward generating a neutralizing antibody response and causing protective immunity (p. 1). Studies had shown that if antibodies were effectively directed toward the spike protein, they would inhibit a virus from entry into host cells. Research of Tai et al. (2020) showed that the spike (S) protein has a significant role in prevention of viral attachment and entry into the cell. This key role of the spike (S) protein became the focus of vaccine research into prevention of viral infection.
This key role of the spike (S) protein became the focus of vaccine research into prevention of viral infection.Once the genetic code for SARS-CoV-2 was released in January 2020 (Branswell, 2021b) the pathway was set for development of various vaccine technologies, including the new mRNA vaccines. Before discussing mRNA vaccines in detail, we provide here a summary of some of the technology platforms being developed alongside the mRNA vaccines.
Concurrent Development of Vaccine Technology Platforms
Johnson & Johnson’s Janssen® COVID-19 vaccine is designed as a recombinant, replication-incompetent human adenovirus Type 26 vector (Janssen, 2021). This design has the capability to enter a human cell and promote generation of SARS-CoV-2 spike (S) antigen without being able to replicate within humans. This vaccine is authorized under the Emergency Use Authorization (EUA) as a single-dose vaccine and produces an immune response protecting against COVID-19 in individuals 18 years of age and over. It requires storage in a refrigerator at 36 to 46 degrees Fahrenheit and should not be frozen (Janssen, 2021). Persons receiving this vaccine are eligible for a booster dose at least two months after the primary dose (Immunize.org., 2021).
The AstraZeneca® COVID-19 vaccine is a viral vector vaccine, like the Janssen® vaccine, using a chimpanzee adenovirus to carry spike proteins into cells and create an immune response. AstraZeneca® vaccines are stored in a regular refrigerator (36–46 degrees Fahrenheit) and should not be frozen (Healthline, 2021). Vaccines stored in a regular refrigerator are an advantage in parts of the world where equipment capable of producing very low storage temperatures is either too expensive or not available.
Vaccines stored in a regular refrigerator are an advantage in parts of the world where equipment capable of producing very low storage temperatures is either too expensive or not available.Two other vaccines supported by Operation Warp Speed use a fully formed spike protein of SARS-CoV-2 attached to an adjuvant. These vaccines stimulate the immune system of vaccinated individuals but cannot cause a COVID-19 infection. These are the Sanofi/GSK® and the Novavax® vaccines. The Sanofi/GSK® vaccine used an adjuvant developed by GSK and a recombinant protein-based vaccine based on a baculovirus commonly found in moths. Development has been delayed after initial results were not as expected but the vaccine is hoped to be considered for regulatory approval by the end of 2021 (Zimlich, 2021a). The Novavax® vaccine uses a custom-designed spike protein to mimic the SARS-CoV-2 virus spike. This two-dose vaccine creates an antibody that will block the virus from binding to cells and preventing infection (Zimlich, 2021b). The Novavax® vaccine is designed for adults 18 years and older and an EUA application was submitted to the FDA in February 2022 (FDA, 2022; Jetelina, 2022). It is designed for adults 18 and older and plans are to apply for authorizations in late 2021.
Messenger RNA (mRNA) Vaccines
Messenger RNA (mRNA) vaccines were only becoming familiar to the public in early 2021. They were so new that many have questioned their safety or speculated that the announced vaccines were not properly tested. This correlates with public concern about the safety of the COVID-19 mRNA vaccines announced in late 2020 that may have affected the willingness of Americans to get vaccinated (Bendau, Plag, Petzold, & Strohle, 2021). As reported by Bendau et al. (2021), there is a potential level of mistrust concerning the rapid development of the vaccines along with undocumented potential side effects and adverse events.
Messenger RNA (mRNA) vaccines were only becoming familiar to the public in early 2021.If one looks at the history of the actual development cycle of mRNA vaccines, one might begin to question whether the technology really is that new. What is the “rest of the story,” to borrow a tagline from Paul Harvey the veteran radio commentator (Watson, 2021). When did work on mRNA vaccines really start? A search of the literature reveals that Wolff et al. (1990) described the first successful experiment of injecting, in vitro mRNA into mouse muscle (Maruggi, Zhang, Li, Ulmer, & Yu, 2019), resulting in the desired protein being encoded within living tissue (Verbeke, Lentacker, De Smedt, & Dewitte, 2019).
Subsequently, Martinon et al. (1993) reported that mRNA transported by liposomes encoded the influenza nucleoprotein to produce cytotoxic T Lymphocytes (Verbeke et al., 2019). Conry et al. (1995) then showed that mRNA can induce anti-tumoral antibody responses, and Hoerr, Obst, Rammensee, & Jung (2000) described how injections with viral or cancer antigen encoding mRNA produced antigen specific immune responses. As concluded by Verbeke et al. (2019), the new technology facilitates rapidly designing mRNA to effectively create antigens and to initiate immunity to combat infectious diseases, which often rapidly mutate, requiring a shorter vaccine development time.
It should also be noted (CDC, 2022a) that mRNA vaccines have been studied for use in influenza, Zika, rabies, and cytomegalovirus (CMV) vaccines prior to use for development of COVID-19 vaccines. Several reasons for the interest in mRNA vaccines include not using infectious particles for penetrating the nuclear membrane; not generating infectious particles in the host cells; and producing complex antigens to promote an immune response. The development of mRNA vaccines to combat COVID-19 infection may be an indication that the days of a vaccine on demand may be here (Maruggi et al, 2019).
...mRNA vaccines have been studied for use in influenza, Zika, rabies, and cytomegalovirus (CMV) vaccines prior to use for development of COVID-19 vaccines.As described by Maruggi (2019) after an antigen has been identified, “the gene is sequenced, synthesized and cloned into the DNA template plasmid” (p. 2). From this point, the mRNA is transcribed (in vitro) and delivered into the target subject. Then by normal cellular processes the desired antigen is produced and starts the process of creating the required humoral and cellular responses. In creation of vaccines for COVID-19, by a process called self-amplification of the mRNA, the resulting vaccine not only generates the immunogenic antigen, but the viral replication machinery required to produce high levels of antigen expression. See Figure for a detailed explanation.
Figure.
Schematic Representation of mRNA Vaccines and Expression of Antigen Expression
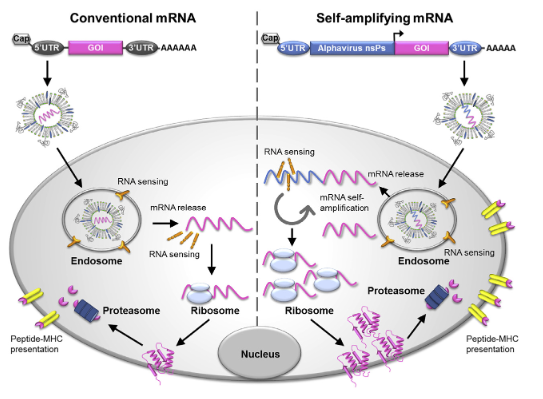
Maruggi et al. (2019), Used with permission
Conventional mRNA carries the coding sequence of the antigen of interest (GOI) flanked by 50 and 30 UTRs, a terminal 50 cap structure, and a 30 poly(A) tail. Once delivered into the cell and released from the endosome into the cytoplasm, the mRNA is translated immediately. The self-amplifying mRNA is often derived from the genome of positive-sense single-stranded RNA viruses, such as alphaviruses. It encodes both the antigen of interest and viral nonstructural proteins (nsPs) required for intracellular RNA amplification and high levels of antigen expression. The self-amplifying mRNA can direct its self-amplification to generate RNA intermediates and many copies of antigen-encoding subgenomic mRNA, producing high levels of the encoded antigen. Both conventional mRNA and self-amplifying mRNA vaccines require a delivery system for cell uptake, usually by endocytosis, which is followed by unloading of mRNA cargo from the endosome into the cytosol, where translation and protein processing for MHC presentation occur. Once delivered in the cell, the mRNA is almost immediately sensed by pattern recognition receptors (PRRs) in the endosome and in the cytoplasm. PRRs such as Toll-like receptors TLR3, TLR7, and TLR8 are localized in the endosome, and cytosolic sensors such as RIG-I, MDA5, PKR, and OAS also recognize double-stranded and single-stranded RNAs in the cytoplasm. GOI, gene of interest; MHC, major histocompatibility complex; nsPs, nonstructural proteins. Maruggi et al. (2019 p. 3).
As summarized by Maruggi et al. (2019), this method of self-amplification on an mRNA platform has the capability to create single- or multiple-antigen vaccines at low dosages producing high antigen response through a robust adjuvant effect. Among the benefits of this technology are reducing the development time and costs of new vaccines and allowing rapid response to new infectious threats to the public. Since the 2019 Maruggi et al. article was published, further research has established that mRNA vaccines are safe and effective.
The CDC (2022a) describes the workings of a COVID-19 mRNA vaccine in this way: After the vaccine is injected into the upper arm, the muscle cells make the desired protein, the spike, on the outside of the COVID-19 virus. With the newly created protein in place, normal cellular processes will degrade the mRNA instructions and eliminate them, just as it does all mRNA proteins. With production of many copies of the protein “spike,” present on the outside of the virus, the immunized person is protected against future infection from the virus. This process is completed without a risk of serious infection from the COVID-19 virus since no parts of a living virus have been introduced to the body.
The key is the process of self-amplification. Not only does the mRNA encode the antigen of the protein “spike” but it also contains the viral replication machinery that will lead to high levels of antigen production (See Figure). This self-amplification is conducted within the cytoplasm of the cell, from low doses of vaccine for an extended period, without entering the nucleus.
mRNA Mode of Action
COVID-19 mRNA vaccines act in multiple waysCOVID-19 mRNA vaccines act in multiple ways (Verbeke et al, 2021). Along with promoting B-cells to produce neutralizing antibodies, macrophages, CD8+ and CD4+ T-cells appear to protect against the SARS-CoV-2 infection. Additionally, memory T-cells in the upper airways appear to limit and shorten duration of disease. As reported by Verbeke et al. (2021), a coordinated response of CD4+T cells, CD8+ T cells and antibody responses in COVID-19 patients correlates with milder disease versus the development of significant disease.
Types of mRNA Vaccines
...these are indicators the body is reacting to the vaccine while building protection...Currently there are two types of mRNA vaccines authorized for use in the United States. The Pfizer-BioNTech® vaccine (BNT162b2), 30 ug per dose, given as 2 doses 21 days apart for persons 12 years of age and over was approved by the U. S. Food and Drug Administration (FDA) on August 23, 2021 (FDA, 2021); and the Moderna® COVID-19 vaccine (Spikemax), 100 ug per dose given 28 days apart for persons 18 years of age and over (CDC, 2022a) was approved on January 1, 2022 by the FDA (FDA, 2022). Both vaccines have typical local side effects (pain, redness and swelling) as well as systemic symptoms (e.g., tiredness, headache, muscle pain, chills, fever, nausea) (CDC, 2022a; 2022c). The CDC comments that these are indicators the body is reacting to the vaccine while building protection, and any discomfort will fade away in a few days. In general, adverse events were more likely to be reported after the second dose (CDC, 2022i).
COVID-19 Variants
Like any virus, the COVID-19 virus can mutate over time. According to the CDC (2022f), the more the virus spreads, the higher chance that it will mutate. This means that the best way to reduce the occurrence of mutations is to protect oneself and those around them by getting vaccinated.
There have been several of variants in circulation during the pandemic (CDC, 2022f). The Alpha variant, commonly known as B.1.1.7 was first identified in the United Kingdom. Others are the Beta variant (B.1.351) identified in South Africa; Gamma (Pl) identified in Japan and Brazil; and Delta (B.1.617.2) identified in India, and the newest is the Omicron variant, first reported from South Africa in November 2021. All spread faster than the original COVID19 virus. Currently the two main variants of concern (VOC) for COVID-19 are the Delta and the Omicron variant – comprising of several variants, some Omicron variants are of concern (WHO, 2021). As of February 23, 2022 (CDC, 2022f) there are five lineages of Omicron, and they are predicted to comprise 100% of all identified cases if they have not already done so.
For a more complete review about the proportions of currently circulating variants, please consult CDC COVID Data Tracker (CDC, 2022d). This site is regularly updated ; the latest update was February 25, 2022.
Many reports cite that the Pfizer® and Moderna® vaccines are effective against the Delta variant (Sanderson, 2021), but the protection wanes over time although the data show existing mRNA vaccines still reduce new infections. For instance, Morbidity and Mortality Weekly Reports (MMWR) published in August 2021 noted that two doses of mRNA vaccines were 74.7% effective against infection among nursing home residents from March-May 2021 (Nanduri et al., 2021) and May 3 – July 25, 2021 (Rosenberg et al., 2021) The overall age-adjusted vaccine effectiveness in New York was stable (91.9%-95.3%) age-adjusted effectiveness for all New York residents declined from 91.7% to 79.8% (Rosenberg et al., 2021). In the discussion, an increase in Delta variant infections may be a cause, but further research is pending.
A recent study (Schmitz, et al., 2021) noted that after collecting 13 types of antibody-producing cells from three subjects previously vaccinated with the Pfizer® COVID-19 vaccine, 12 of them recognized the Alpha and Delta variants, eight recognized all four variants, and only one antibody did not recognize any of the four variants of interest. The researchers commented that this response shows that available antibodies should be capable of neutralizing the variants. More research regarding the COVID-19 variants is planned.
Effectiveness of mRNA Vaccines
Initial phase III trial results of the two mRNA vaccines were very effective. The Pfizer® vaccine prevented COVID-19 disease (up to 95% efficacy) while the Moderna® vaccine was 94.1% effective (Verbeke et al., 2021). These results were comparable with results in Israel where the Pfizer® vaccine was estimated at 97% effective at preventing symptomatic/severe COVID-19 disease and 94% effective against asymptomatic infection (Verbeke et al., 2021).
...it is key to remember that no vaccine is 100% effective and some persons fully vaccinated will become infected ...While the reader may consult many sources with conflicting reports about vaccine effectiveness, it is key to remember that no vaccine is 100% effective and some persons fully vaccinated will become infected (known as “breakthrough infection”) (CDC, 2021a). COVID-19 vaccines are still protective as fully vaccinated persons will likely be infectious for less time than the unvaccinated and the vaccine will protect them against serious illness and death (CDC, 2021a).
A point of note, according to Jaleesa Baulkman (2021), those who have received their first or second mRNA vaccine and experience breakthrough disease are two times more likely to be asymptomatic and approximately two-thirds less likely to be hospitalized. Baulkman also described that likelihood of severe symptoms dropped after one dose and those vaccinated persons, especially those 60 years of age and older, were more likely to express symptoms.
Late News about the Pfizer-BioNTech® COVID-19 Vaccine
The FDA (2021) approved the Biologics License Application (BLA) for COMRINATY®, made by Pfizer for BioNTech®. The approval is for a 2-dose series in individuals 16 years of age and older and includes an Emergency Use Authorization (EUA) to prevent COVID-19 in individuals 12 through 15 years as well as a third dose for individuals 12 years and older who are immunocompromised. With the vaccine shown to be 91% effective in preventing COVID-19 in clinical trials, it is hoped this announcement will provide reassurance that the Pfizer-BioNTech (COMRINATY®) vaccine is safe and effective and will encourage more citizens to present for vaccination. On October 29, 2021, the Pfizer-BioNTech® vaccine was authorized by the Advisory Committee on Immunization Practice (ACIP) for individuals 5 through 11 years of age as two-dose primary series 3 weeks apart under an Emergency Use Authorization (EUA) (CDC, 2022h). Readers should note the COMRINATY® vaccine made by Pfizer for BioNTech® is the only COVID-19 vaccine approved for children ages 5-17 years of age (CDC, 2022h).
Some recent changes include a recommendation for a 3-dose series of mRNA vaccine for immunocompromised patients ages 5 and older, followed by a booster dose for ages 12 and older (CDC, 2022j). It should be noted that the CDC prefers the use of mRNA vaccines rather than the Janssen COVID-19 vaccine for primary and booster doses. (CDC, 2022j). And a booster dose is recommended for those 12 years and older with the timing based upon the vaccine product and immunocompetence of the person (CDC, 2022j).
Another consideration for people ages 12 years and older is the recommendation of an 8-week interval between the first and second dose of mRNA vaccine (CDC, 2022j). This is especially focused on males ages 12 through 39 who are at the highest risk for myocarditis after the second does. Research shows a production of a higher level of antibodies while a lower risk for developing myocarditis with this longer interval (CDC, 2022j).
Booster Doses of COVID-19 Vaccines
On August 27, 2021, the CDC recommended (CDC, 2022b) a booster dose of COVID-19 vaccine for patients who are moderately to severely compromised. The rationale is that these persons may not generate an adequate immune response as compared to those who are not immunocompromised. Then, on September 23, 2021, during a virtual ACIP Meeting the Committee Members voted to recommend, unanimously, a third booster dose for adults 65 years of age and older as well as for those ages 50 to 64 with high-risk medical conditions (the vote was 13 – 2). Subsequently, Dr. Rochelle Walensky, CDC Director, signed off on the first two ACIP votes, then made her own recommendation to include those 18 to 64 years of age who are health-care workers or other occupation considered high risk of exposure (Associated Press, 2021)
As of February 2, 2022, the CDC recommends everyone 12 years and older, and at least 5 months after completing your primary COVID-19 vaccination series with a Pfizer-BioNTech® vaccine should receive a booster shot (CDC, 2022e). Anyone 18 years and older who received the Moderna® vaccine should receive a booster at least 5 months after completing the primary COVID-19 vaccination series (CDC, 2022e). Finally, anyone 18 years and older who received a single dose of Johnson & Johnson’s Janssen vaccine should receive a booster at least 2 months after that first shot (CDC, 2022e).
Additionally, a person may preferentially choose which COVID-19 vaccine to receive for the booster. CDC recommendations now allow for a mix and match dosing for booster shots (CDC, 2022e).
The discussion of booster doses has generated many questions from the public.The discussion of booster doses has generated many questions from the public. For example – is vaccine effectiveness (VE) waning over time? Are they needed at this time? What is the effectiveness of current vaccines against the new variants that are arising? Might it be possible that the initial series of COVID-19 vaccines should be three (3) doses (instead of two) to generate the maximum effectiveness in all persons? Another question is should a third dose be called a booster or be called part of a 3-dose series, as noted by Dr. Stanley Plotkin at the August 30, 2021, virtual ACIP meeting. (Branswell, 2021b).
These are significant questions of concern, and extensive research and discussion are being conducted at this time – not only at the CDC and other agencies but within the ACIP. More information will be published as it becomes available, so stay tuned for further developments. At this writing there is speculation that a second booster is being discussed. A recent MMWR authored by Thompson, M., et al., (2022) reports that receipt of COVID-19 vaccine given as a third booster dose was highly effective against the Delta and Omicron variants in preventing serious disease, hospitalization, and deaths.
Readers seeking sites containing updated information about COVID-19 vaccine may wish to become familiar with and bookmark the following sites related to COVID-19 educational information. Two examples are Vaccine for COVID-19 (2021b) for basic information about COVID-19 vaccines (CDC, 2021b) or COVID Data Tracker (CDC, 2022g) where the readers can explore the COVID Data Tracker and other information relative to the pandemic. A third site recommended is the Immunization Action Coalition site: Vaccines: COVID-19 (Immunize.org, 2022). Again, it is a good idea to become familiar with these resources since they can be helpful in teaching patients and their families.
Further Questions
There are still questions to address in future research. According to Verbeke et al. (2021) a key question is how long immunity will last. Also unanswered is the effectiveness of the lipoprotein carrier in delivering the mRNA into the cell and its translation into the proteins needed for immunogenicity and the reactogenicity of the vaccine (Verbeke et al., 2021). Will any further improvements in the technology be possible? It is felt that more research is needed to identify if a more efficient delivery of mRNA is possible and how it may impact the performance of current vaccines, thus promoting safer and more effective vaccines in the future.
Putting off attendance at events that are a part of life can only delay exposure to the virus.Increased development of the mRNA platform will most likely impact work on Zika, Rabies, Influenza, and respiratory syncytial virus (RSV) as well as oncology applications where the immune system can eliminate tumor cells. A late addition to the discussion comes from Vinay Prasad, MD, MPH with Medpage (2021), who asserted that every person on the planet will be exposed to the COVID-19 virus over the next 10 years and it will be impossible to avoid. One can delay exposure, but eventually will encounter the virus. Putting off attendance at events that are a part of life can only delay exposure to the virus. So, “each of us has to decide how much life we are willing to trade to delay our time to meet the virus” (Prasad, 2021, p. 3).
It will be wise for nurses and other healthcare professionals to monitor the COVID-19 vaccine development literature to remain current on the state of the science. It is important to ensure that COVID-19 developmental literature consulted is from a trusted and valid source. Nurses remain the most trusted healthcare professionals. Information shared by nurses should align with the best available evidence. Whether it is the Delta or Omicron variant or any other variant that may emerge, keeping current is necessary for all healthcare professionals to properly educate patients and families.
Conclusion: Implications for Nurses
Since early 2020 the nursing profession has been challenged by the COVID-19 pandemic. Many questions have arisen, including:
- What is the epidemiology of the virus? How does it cause disease?
- How can it be treated? And now, how will we make most effective use of the new vaccines that are emerging?
- What is the efficacy of these vaccines? Does their effectiveness wane? Should boosters be recommended, and at what intervals?
The nursing and medical professions are provided many new reports daily. Providers are challenged to determine whether information is valid and which reports are merely speculation.
Nurses must focus on consulting with trusted references that provide the best available evidence... It is the responsibility of all nurses to keep current on developments about basic knowledge of the virus, effective treatments, and vaccines to prevent infection and development of disease symptoms. Nurses must focus on consulting with trusted references that provide the best available evidence so they can communicate this knowledge to patients. If nurses are up to date on all these topics, they must proactively cultivate their ability to effectively communicate with patients and families, answer their questions, and lead them toward informed decisions to prevent acquiring the virus or managing recovery. This virus may be with us for a long time, so it is incumbent on nurses to educate patients so they can live longer and healthier lives.
Authors
Chad Rittle DNP, MPH, RN FAAOHN
Email: crittle@chatham.edu
ORCID ID: 0000-0003-3893-1209
Dr. Chad Rittle is an Associate Professor of Nursing at Chatham University where he teaches in the RN-BSN program with an emphasis in community/public health, immunizations, and cross-cultural nursing. Dr. Rittle has worked many years in public health with the Commonwealth of PA as well as in long-term care. He volunteered to serve at five COVID-19 vaccine clinics in Western PA during Spring of 2021 as either a vaccine administrator or observer. He has served as the ANA Liaison to the Advisory Committee on Immunization Practice (ACIP) since 2014 and actively advocates and educates towards universal vaccination of all adults.
Lora Walter, DNP, RNC-NIC
Email: lwalter@chatham.edu
ORCID ID: 0000-0003-3893-1209
Dr. Lora Walter is an Assistant Professor of Nursing at Chatham University where she coordinates the RN-BSN and Pathways to Nursing Programs. She teaches in the MSN and RN-BSN programs with a focus on nursing education and women’s health. As a neonatal certified registered nurse, Dr. Walter worked in a level III neonatal intensive care unit (NICU) for 8 years before moving to academia. As a Medical Reserve Corps (MRC) volunteer Dr. Walter became actively involved with COVID-19 vaccine administration at the onset of the pandemic. She has advocated for vaccine administration through patient education based on her knowledge and experience working in various clinics.
References
American Journal of Managed Care (AJMC) Staff. (2021, December 23). A timeline of COVID-19 developments in 2021. News. https://www.ajmc.com/view/a-timeline-of-covid-19-vaccine-developments-for-the-second-half-of-2021
The Associated Press (AP). (2021, September 24). CDC Director Backs COVID booster plan and makes an additional recommendation. NPR, Health. https://www.npr.org/2021/09/24/1040348413/cdc-director-backscovid-booster-plan-and-makes-an-additional-recommendation
Baulkman, J. (2021, September 1). Breakthrough infections twice as likely to be asymptomatic. Internal Medicine News. https://www.mdedge.com/internalmedicine/article/245429/coronavirus-updates/breakthrough-infections-twice-likely-be
Bendau, A., Plag, J., Petzold, M. B., & Strohle, A. (2021). Covid-19 vaccine hesitancy and related fears and anxiety. International Immunopharmacology, 97, 107724 https://www.doi.org/10.1016/j.intimp.2021.107724
Branswell, H. (2021a, June 30). 12 lessons COVID-19 taught us about developing vaccines during a pandemic. Stat News. https://www.statnews.com/2021/06/30/12-lessons-covid-19-developing-vaccines/
Branswell, H., (2021b, September 2). The debate over COVID-19 vaccine boosters, what to call them, and whether they’re needed. Stat News. https://www.statnews.com/2021/09/02/the-debate-over-covid-19-vaccine-boosters-what-to-call-them-and-whether-theyre-needed/
Centers for Disease Control and Prevention (CDC). (2015). Information about Middle East Respiratory Syndrome (MERS) [fact sheet]. Middle East Respiratory Syndrome (MERS). https://www.cdc.gov/coronavirus/mers/downloads/factsheet-mers_en.pdf
Centers for Disease Control and Prevention (CDC). (2017). SARS basics fact sheet. Severe acute respiratory syndrome (SARS). https://www.cdc.gov/sars/about/fs-sars.html
Centers for Disease Control and Prevention (CDC). (2019). About MERS. Middle East Respiratory Syndrome (MERS). https://www.cdc.gov/coronavirus/mers/about/index.html
Centers for Disease Control and Prevention (CDC). (2021a, August 26). Delta variant: What we know about the science. Your Health. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
Center for Disease Control (CDC). (2021b, September 1). Vaccines for COVID-19. Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/
Centers for Disease Control and Prevention (CDC). (2021c, November 26). CDC statement on B.1.1.529 (Omicron variant) [press release]. CDC Newsroom Releases. https://www.cdc.gov/media/releases/2021/s1126-B11-529-omicron.html
Centers for Disease Control and Prevention (CDC). (2022a, January 4). Understanding mRNA COVID-19 vaccines. Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mRNA.html
Centers for Disease Control and Prevention (CDC). (2022b, January 7). COVID-19 vaccines for moderately to severely immunocompromised people. Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
Centers for Disease Control and Prevention (CDC). (2022c, February 1). Moderna COVID-19 vaccine overview and safety. Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html
Centers for Disease Control and Prevention (CDC). (2022d, February 1). Variant proportions. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
Centers for Disease Control and Prevention (CDC). (2022e, February 2). COVID-19 vaccine booster shots. Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
Centers for Disease Control and Prevention (CDC). (2022f, February 2). What you need to know about variants. Your Health. https://www.cdc.gov/coronavirus/2019-ncov/variants/about-variants.html
Centers for Disease Control (CDC). (2022g, February 7). COVID data tracker. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
Centers for Disease Control and Prevention (CDC). (2022h, January 11). COVID-19 vaccines for children and teens. Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/children-teens.html?s_cid=11368:minimum%20age%20for%20covid%20vaccine:sem.ga:p:RG:GM:gen:PTN:FY21
Centers for Disease Control and Prevention (CDC). (2022i, January 12). Possible side effects of getting a COVID-19 vaccine. Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html
Centers for Disease Control and Prevention (CDC). (2022j, February 22) Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. COVID-19 Vaccination. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#booster-dose
Cohrs, R. (2021, March 2). The Trump administration quietly spent billions in hospital funds on Operation Warp Speed. STAT News. https://www.statnews.com/2021/03/02/trump-administration-quietly-spent-billions-in-hospital-funds-on-operation-warp-speed/
Conry, R. M., LoBuglio, A. F., Wright, M., Sumerel, L., Pike, M. J., Johanning, F., Benjamin, R., Lu, D., & Curiel, D. T. (1995). Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Research, 55(7), 1397–1400. https://cancerres.aacrjournals.org/content/55/7/1397
Healthline. (2021, October 27). What you should know about the AstraZeneca COVID-19 vaccine. COVID-19. https://www.healthline.com/health/adult-vaccines/astrazeneca-vaccine#how-it-works
Healthline.com (2020, April 29). COVID-19 vs. SARS: How do they differ? COVID-19. https://www.healthline.com/health/coronavirus-vs-sars
Hoerr, I., Obst, R., Rammensee, H. G., & Jung, G. (2000). In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. European Journal of Immunology, 30(1), 1–7. https://doi.org/10.1002/1521-4141(200001)30:1%3C1::AID-IMMU1%3E3.0.CO;2-%23
Immunize.org. (formerly Immunization Action Coalition [IAC]). (2022, January 11). Vaccines: COVID-19. Vaccines. https://www.immunize.org/covid-19/
Janssen. (2021). Clinical data for Johnson & Johnson’ Janseen Covid-19 vaccine. About the Vaccine. https://www.janssencovid19vaccine.com/hcp/clinical-data.html#booster-dose
Jetelina, K. (2022, February 4). Two underdog but game changing vaccines: NVX-CoV2373 (Novavax) and Corbevax. Your Local Epidemiologist. https://yourlocalepidemiologist.substack.com/p/two-underdog-but-game-changing-vaccines?utm_source=url
Martinon, F., Krishnan, S., Lenzen, G., Magné, R., Gomard, E., Guillet, J. G., Lévy, J. & Meulien, P. (1993). Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. European Journal of Immunology, 23(7), 1719–1722. https://doi.org/10.1002/eji.1830230749
Maruggi, G., Zhang, C., Li, J., Ulmer, J. B., & Yu, D. (2019). mRNA as a transformative technology for vaccine development to control infectious diseases. Molecular Therapy, 27(4), 757-766. https://doi.org/10.1016/j.ymthe.2019.01.020
Morens, D. M., Breman, J. G., Calisher, C. H., Doherty, P. C., Hahn, B. H., Keusch, G. T., Kramer, L. D., LeDuc, J. W., Monath, T. P., & Taubenberger, J. K. (2020). The origin of Covid-19 and why it matters. The American Journal of Tropical Medicine and Hygiene, 103(3), 955-959. https://doi.org/10.4269/ajtmh.20-0849
Nanduri, S., Pilishvili, T., Derado, G., Soe, M. M., Dollard, P., Wu, H., Li, Q., Bagchi, S., Dubendris, H., Link-Gelles, R., Jernigan, J., Budnitz, D., Bell, J., Benin, A., Shang, N., Edwards, J. R., Verani, J, & Schrag, S. J. (2021, August 27). Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B1.617.2 (Delta) variant – National Healthcare Safety Network, March 1-August 1, 2021. Morbidity and Mortality Weekly Report (MMWR), 70(34), 1163-1166. https://doi.org/10.15585/mmwr.mm7034e3
Novavax. (2022, January 31). Novavax submits request to the U.S. FDA for Emergency Use Authorization of COVID-19 vaccine. Press Releases & Statements. https://ir.novavax.com/2022-01-31-Novavax-Submits-Request-to-the-U-S-FDA-for-Emergency-Use-Authorization-of-COVID-19-Vaccine
Prasad, V. (2021, September 22). Accept it: COVID will be an endemic virus: Everyone will meet with the virus eventually, but doing so safely (while vaccinated) is key [opinion]. MEDPAGE Today. https://www.medpagetoday.com/opinion/vinay-prasad/94646
Rat, P., Olivier, E., & Dutot, M. (2020). SARS-CoV-2 vs. SARS-CoV-1 management: Antibiotics and inflammasome modulators potential. European Review for Medical and Pharmacological Sciences, 24(14), 7880-7885. https://doi.org/10.26355/eurrev_202007_22293
Reuters. (2022, February 28). Novavax expects to apply for full approval of COVID vaccine in H2. Business & Healthcare Pharmaceuticals. https://www.reuters.com/business/healthcare-pharmaceuticals/novavaxs-covid-vaccine-shows-long-term-efficacy-uk-trial-2022-02-28/
Rosenberg, E. S., Holtgrave, D. R., Dorabawila, V., Conry, M., Greene, D., Lutterloh, E., Backenson, B., Hoefer, D., Morne, J., Bauer, U., & Zucker, H. (2021, August 27). New COVID-19 cases and hospitalizations among adults by vaccinating status – New York, May 3-July 25, 2021. Morbidity and Mortality Weekly Report (MMWR), 70(34). 1150-1155. https://doi.org/10.15585/mmwr.mm7034e1
Salvatori, G., Luberto, L., Maffei, M., Aurisicchio, L., Roscilli, G., Palombo, F., & Marra, E. (2020). SARS-CoV-2 SPIKE PROTEIN: An optimal immunological target for vaccines. Journal of Translational Medicine, 18, 222. https://doi.org/10.1186/s12967-020-02392-y
Sanderson, K. (2021, August 19). COVID vaccines protect against Delta, but their effectiveness wanes. Nature. https://doi.org/10.1038/d41586-021-02261-8
Schmitz, A. J., Turner, J. S., Liu, Z., Zhou, J. Q., Aziati, I. D., Chen, R. E., Joshi, A., Bricker, T. L., Darling, T. L., Adelsberg, D. C., Altomare, C. G., Alsoussi, W. B., Case, J. B., VanBlargan, L. A., Lei, T., Thapa, M., Amanat, F., Jeevan, T., Fabrizio, T., …& Ellebedy, A. H. (2021). A vaccine-induced public antibody protects against SARS-CoV-2 and emerging variants. Immunity, 54(9), 2159-2166. https://doi.org/10.1016/j.immuni.2021.08.013
Tai, W., He, L., Zhang, X., Pu, J., Voronin, D., Jiang, S., Zhou, Y., & Du, L. (2020). Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cellular & Molecular Immunology, 17, 613-620. https://doi.org/10.1038/s41423-020-0400-4
Thompson, M. G., Natarajan, K., Irving, S. A., Rowley, E. A., Griggs, E. P., Gaglani, M., Klein, N. P., Grannis, S. J., DeSilva, M. B., Stenehjem, E., Reese, S. E., Dickerson, M., Naleway, A. L., Han, J., Konatham, D., McElvoy, C., Rao, S., Dixon, B. E., Dascomb, K…Ong, T. C. (2022, January 28). Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance — VISION Network, 10 States, August 2021–January 2022. MMWR Morbidity and Mortality Weekly Report, 71(4), 139-145. http://dx.doi.org/10.15585/mmwr.mm7104e3
U.S. Department of Defense (DoD). (2020). Trump administration announces framework and leadership for ‘Operation Warp Speed [press release]. News. https://www.defense.gov/News/Releases/Release/Article/2310750/trump-administration- announces-framework-and-leadership-for-operation-warp-speed/
U. S. Food & Drug Administration (FDA). (2021, August 23) FDA approves first COVID-19 vaccine: Approval signifies key achievement for public health [press release]. FDA Newsroom. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
U. S. Food & Drug Administration (FDA). (2022, January 31) Coronavirus (COVID-19) update: FDA takes key action by approving second COVID-19 vaccine [press release]. FDA Newsroom. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine
U. S. Government Accountability Office (GAO). (2021, February 11). Operation warp speed. Accelerated COVID- 19 vaccine development status and efforts to address manufacturing challenges [press release]. Reports & Testimonies. https://www.gao.gov/products/gao-21-319
Verbeke, R., Lentacker, I., De Smedt, S. C., & Dewitte, H. (2021). The dawn of mRNA vaccines: The COVID-19 case. Journal of Controlled Release, 333, 511-520. https://doi.org/10.1016/j.jconrel.2021.03.043
Verbeke, R., Lentacker, I., De Smedt, S., & Dewitte, H. (2019). Three decades of messenger RNA vaccine development. Nano Today, 28, 100766. https://doi.org/10.1016/j.nantod.2019.100766
Watson, C. (2021, October 9). The rest of the story: Paul Harvey, conservative talk radio pioneer. NPR, Health. https://www.npr.org/2014/10/09/354718833/the-rest-of-the-story-paul-harveyconservative-talk-radio-pioneer
World Health Organization (WHO). (2020, March 11). WHO Director-General’s opening remarks at the media briefing on COVID-19. Who Director-General. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
Wolff, J. A., Malone, R. W., Williams, P., Chong, W., Acsadi, G., Jani, A., & Felgner, P. L. (1990). Direct gene transfer into mouse muscle in vivo. Science, 247(4949), 1465–1468. https://www.jstor.org/stable/2874228
Wu, F., Zhao, S., Yu, B., Chen, Y. M., Wang, W., Song, Z. G., Hu, Y., Wu, Z. W., Tian, J. H., Pei, Y. Y., Yuan, M. L. Zhang, Y. L., Dai, F. H., Liu, Y., Wang, Q. M., Zheng, J. J., Xu, L., Holmes, E. C., & Zhang, Y. Z. (2020). A new coronavirus associated with human respiratory disease in China. Nature, 579, 265-269. https://doi.org/10.1038/s41586-020-2008-3
Zimlich, R. (2021a, June 1). An overview of the Sanofi/GSK COVID-19 vaccine. Infectious Diseases. https://www.verywellhealth.com/sanofi-gsk-covid-19-vaccine-5093295
Zimlich, R. (2021b, June 18). An overview of the Novavax COVID-19 vaccine. Infectious Diseases. https://www.verywellhealth.com/novavax-covid-19-vaccine-5093292

